Inorganic Chemistry MCQ on Periodicity of Elements
Inorganic Chemistry MCQ on Periodicity of Elements
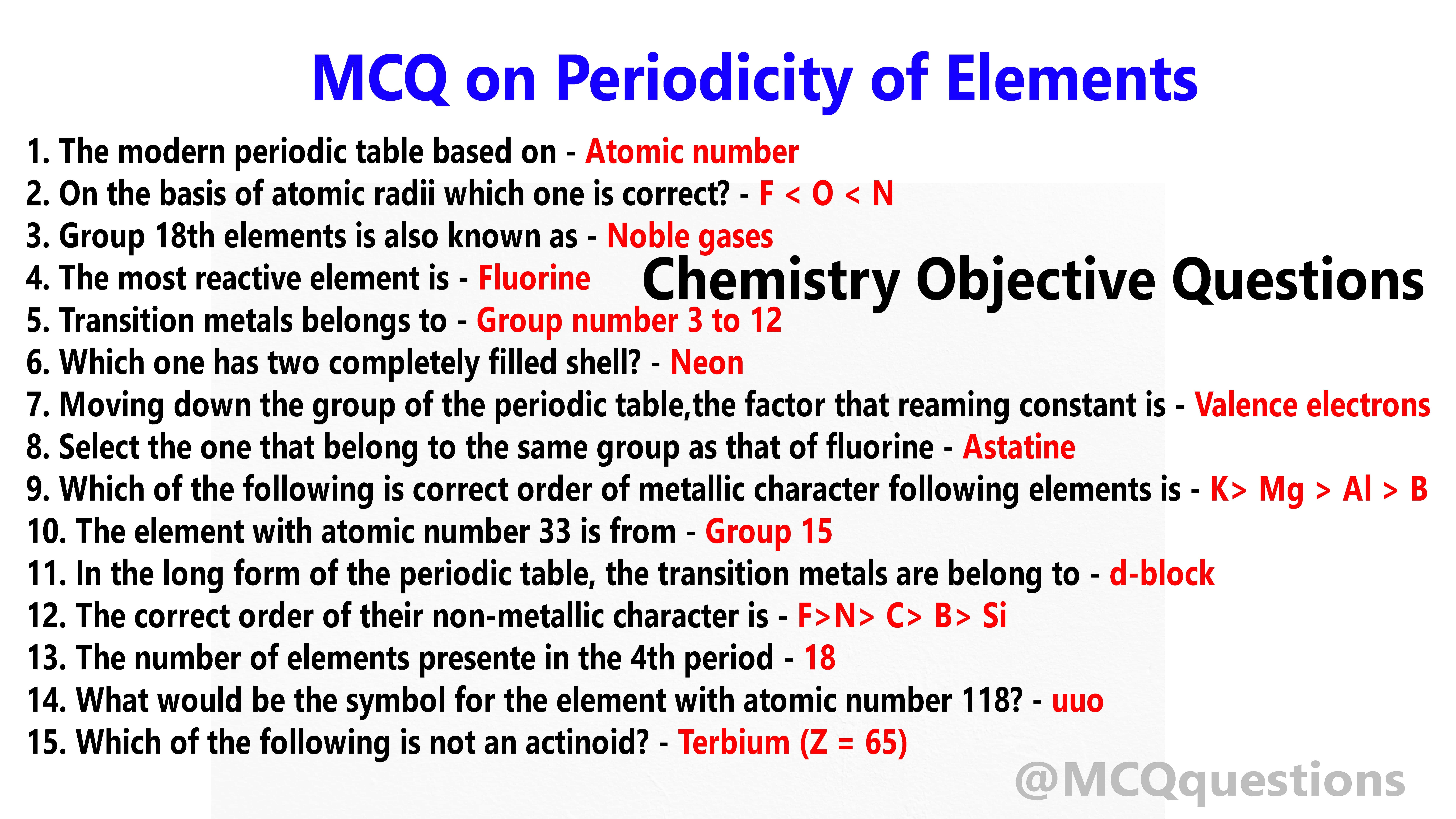
1. The modern periodic table based on
(a) Atomic mass
(b) Atomic number
(c) Number of nucleons
(d) All of these
Answer b) Atomic number
2. The changes in electro positivity of elements on moving from left to right in a periodic table is
(a) Increase
(b) Decreases
(c) First increases than decreases
(d) First decreases than increases
Answer (b) Decreases
3. What is the position of the element in moder periodic table having electronic configuration 2, 8, 4. ?
(a) 4th group
(b) 2nd group
(c) 14th group
(d) 18th group
Answer (c) 14th group
4. On the basis of atomic radii which one is correct?
(a) O < F < N
(b) N < F < O
(c) O < N < F
(d) F < O < N
Answer (d) F < O < N
5. Group 18th elements is also known as
(a) Noble gases
(b) Alkali metals
(c) Alkali earth metals
(d) Halogens
Answer (a) Noble gases
6. The most reactive element is
(a) Oxygen
(b) Sodium
(c) Fluorine
(d) Magnesium
Answer (c) Fluorine
7. Transition metals belongs to
(a) Group number 1 to 2
(b) Group number 13 to 18
(c) Group number 3 to 12
(d) Group number 1 to 8
Answer (c) Group number 3 to 12
8. Which one has two completely filled shell?
(a) Helium
(b) Neon
(c) Calcium
(d) Boron
Answer (b) Neon
9. Moving down the group of the periodic table,the factor that reaming constant is
(a) Atomic radius
(b) Metallic character
(c) Number of shells in the atom
(d) Valence electrons
Answer (d) Valence electrons
10. Which of the following is not characteristic of d-block elements?
(a) They form coloured complexes
(b) They are generally paramagnetic
(c) Their ionization energies are very high
(d) They show variable oxidation states
Answer (c) Their ionization energies are very high
11. Choose the wrong one.
(a) The properties of elements are the periodicity of their atomic number.
(b) metallic elements are more in number than non-metallic elements
(c) The first IE of elements along a period do not vary .
(d) For transition elements, the last electron enters into (n-3)d-subshell.
Answer (d) For transition elements, the last electron enters into (n-3)d-subshell
12. In the sixth period of the periodic table, we have
(a) Two s-block and six p-block elements
(b) Fourteen f-block elements
(c) ten d-block elements
(d) All the above.
Answer (d) All the above.
13. In the sixth period of the periodic table, 14 elements are placed in the third group. They are known as
(a) Alkali metals
(b) Rare earths
(c) Rare gases
(d) Alkaline carth metals
Answer (b) Rare earths
14. Select the one that belong to the same group as that of fluorine
(a) Astatine
(b) Cesium
(c) Radium
(d) Rubidium.
Answer (a) Astatine
15. Which of the following is correct order of metallic character following elements is
(a) B > Al> Mg > K
(b) Al> Mg > B> K
(c) Mg > Al> K > B
(d) K> Mg > Al > B.
Answer (d) K> Mg > Al > B.
16. Tik the incorrect statement in relation to ionization enthalpy?
(a) lonization enthalpy increases for each successive electron.
(b) The greatest IP found in core noble gas configuration.
(c) End of outer electron is marked by a big jump in ionization enthalpy.
(d) Removal of electron from lower n value is easier than from higher n value orbital.
Answer (d) Removal of electron from lower n value is easier than from higher n value orbital
17. The element with atomic number 33 is from
(a) Group 13
(b) Group 15
(c) Group 14
(d) Group 16.
Answer (b) Group 15
18. In the long form of the periodic table, the transition metals are belong to
(a) s-block
(b) p-block
(c) d-block
(d) f-block.
Answer (c) d-block
19. The correct order of their non-metallic character is
(a) B> C> Si>N> F
(b) Si > C> B>N> F
(c) F>N> C> B> Si
(d) F>N> C> Si > B
Answer (c) F>N> C> B> Si
20. Given energy of ground state electron of hydrogen -2.18 10-18 J. Calculate the ionization energy of atomic hydrogen in terms of J mol.
(a) 2.313 x 10⁶ J/mol
(b) 1.313 x 10⁶ J/mol
(c) 2.313 x 10-⁶ J/mol
(d) 4.313 x 10-⁶J/mol.
Answer (b) 1.313 x 10⁶ J/mol
21. Which of the following statements is incorrect?
(a) periodic table contains 7 period and 18 groups.
(b) The d-block has 8 columns.
(c) Each block contains a number of columns equal to the number of electrons.
(d) The block indicates l value for the last subshell.
Answer (b) The d-block has 8 columns.
22. The number of elements presente in the 4th period
(a) 8
(b) 10
(c) 18
(d) 13.
Answer (c) 18
23. Which of the following statements are incorrect?
(a) Helium is a noble gas.
(b) Chlorine has less negative EA than fluorine.
(c) s and p elements are Representative elements.
(d) In any period, atomic radius of alkali metals is the highest.
Answer (b) Chlorine has less negative EA than fluorine.
24. What would be the symbol for the element with atomic number 118?
(a) uuq
(b) uup
(c) uus
(d) uuo.
Answer (d) uuo.
25. Considering the elements F. CI, O and N, the correct order of oxidizing property is
(a) F> CI> 0>N
(b) F>O>CI > N
(c) CI>F>O>N
(d) O> F>N> CL
Answer (b) F>O>CI > N
26. Which of the following is not an actinoid?
(a) Curium (7- 96)
(b) Uranium (Z-92)
(c) Californium (Z = 98)
(d) Terbium (Z = 65).
Answer (d) Terbium (Z = 65).
27. Select the incorrect statement.
(a) Elements of group 16 are known as chalcogens.
(b) Actinoid elements are radioactive.
(c) Electronic configuration na belongs to s-block elements.
(d) Zn show most of the properties of transition elements.
Answer (d) Zn show most of the properties of transition elements.
28. Correct increasing metallic character order is
(a) P< Si < Na< Be < Mg
(b) Be< Mg<P < Na < Si
(c) Si <Be> Mg < Na < P
(d) P<Si < Be Mg < Na.
Answer (d) P<Si < Be Mg < Na.
29. Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
(a) Valence principal quantum number (n)
(b) Nuclear charge (Z)
(c) Nuclear mass
(d) Number of core electrons.
Answer (c) Nuclear mass
Also You Can Read Inorganic Chemistry MCQ