Inorganic Chemistry MCQ on Chemical Bonding
Inorganic Chemistry MCQ on Chemical Bonding
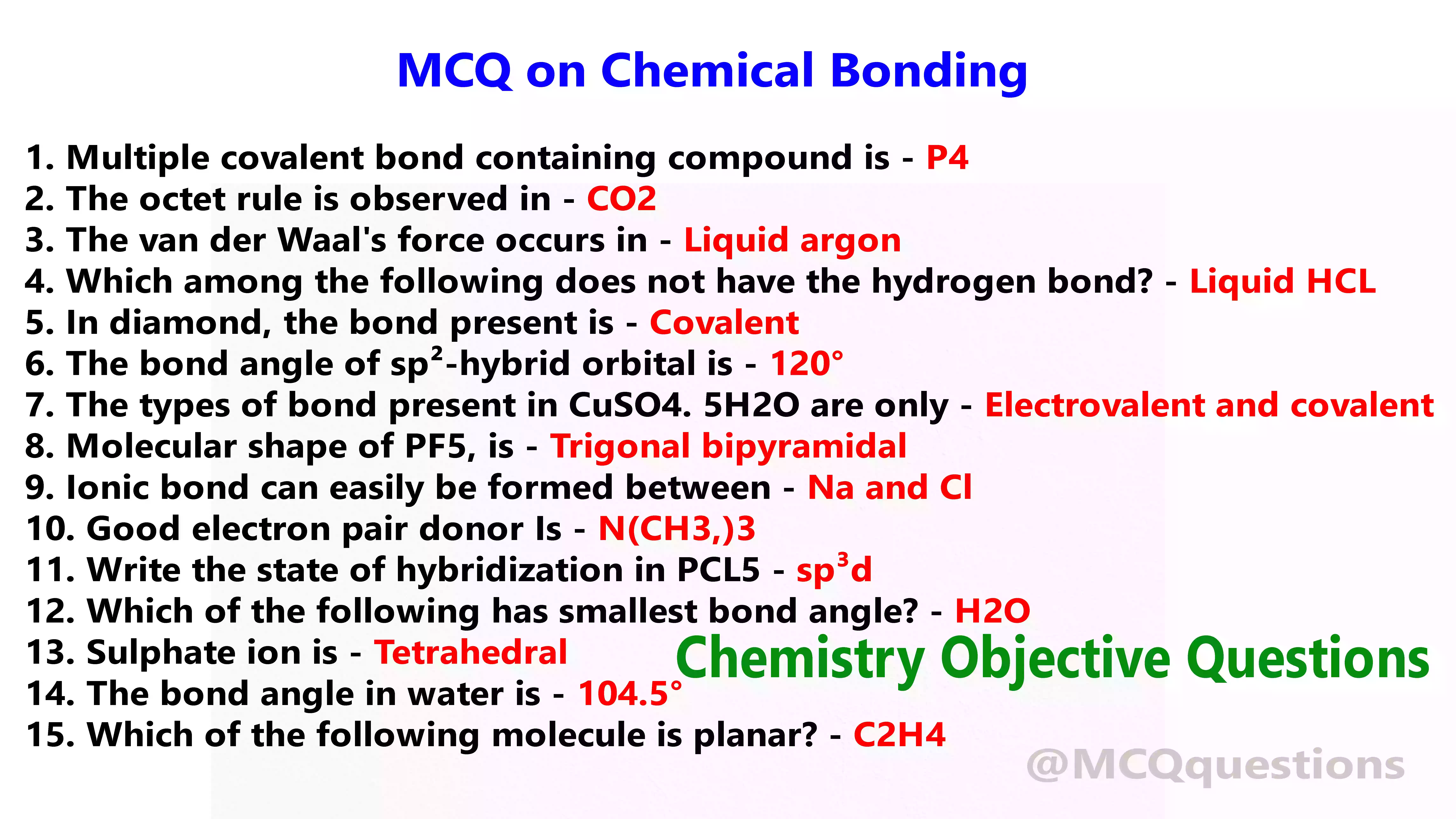
1. Multiple covalent bond containing compound is
(a) CH4
(b) S8
(c) P4
(d) H2
Answer (c) P4
2. The octet rule is observed in
(a) PCI5
(b) CO2
(c) BCI3
(d) SF6
Answer (b) CO2
3. The van der Waal’s force occurs in
(a) Liquid HF
(b) Liquid NH,
(c) Ice
(d) Liquid argon
Answer (d) Liquid argon
4. The compound containing coordinate bond is
(a) SO3
(b) H2SO4
(c) O3
(d) all of these
Answer (d) all of these
5. Which among the following does not have the hydrogen bond?
(a) Water
(b) Liquid NH
(c) Phenol
(d) Liquid HCL
Answer (d) Liquid HCL
6. Carbon tetrachloride has no dipole moment, because
(a) Similar sizes of carbon and chlorine atoms
(b) It is symmetrical tetrahedral structure
(c) It is planer structure
(d) None of these.
Answer (b) It is symmetrical tetrahedral structure
7. The tetrahedral structure is possessed by
(a) BF4-
(b) CH4
(c) NH4+
(d) all of these
Answer (d) all of these
8. In diamond, the bond present is
(a) Hydrogen
(b) Covalent
(c) Electrovalent
(d) Coordinate bond.
Answer (b) Covalent
9. The bond angle of sp²-hybrid orbital is
(a) 105°
(b) 120°
(c) 180°
(d) 109°28′
Answer (b) 120°
10. The types of bond present in CuSO4. 5H2O are only
(a) Electrovalent and covalent
(b) Electrovalent and coordinate covalent
(c) Electrovalent, covalent and coordinate-covalent
(d) Electrovalent, covalent and hydrogen.
Answer (a) Electrovalent and covalent
11. Molecular shape of PF5, is
(a) square planar
(b) Trigonal bipyramidal
(c) Tetrahedral three-dimensional
(d) Octahedral three-dimensional.
Answer (b) Trigonal bipyramidal
12. Ionic bond can easily be formed between
(a) H and O
(b) H and Cl
(c) H and N
(d) Na and Cl
Answer (d) Na and Cl
13. What happend in presence of more electronegative atom to the strength of ionic bond?
(a) Decreases
(b) Increases
(c) remains same
(d) Any one of these.
Answer (b) Increases
14. If we consider the ammonia molecule to be sp³ hybridised. the lone pair will occupy
(a) An s-orbital
(b) A p-orbital
(c) A sp²-hybridised orbital
(d) A sp³-hybridised orbital
Answer (d) A sp³-hybridised orbital
15. Strongest hydrogen bonds in vapour phase are found in
(a) HF—– HF
(b) HCI—–HCI
(c) HF—- HCI
(d) HF—-HI.
Answer (a) HF----- HF
16. Good electron pair donor
Is
(a) P(CH3)3
(b) PH3
(c) N(CH3,)3
(d) NH3
Answer (c) N(CH3,)3
17. Write the state of hybridization in PCL5
(a) dsp³
(b) sp³d
(c) dsp²
(d) sp³d²
Answer (b) sp³d
18. Which of the following has smallest bond angle?
(a) H2O
(b) NH3
(c) CH4
(d)CO2
Answer (a) H2O
19. Sulphate ion is
(a) square planar
(b) Pyramidal
(c) Rhombic
(d) Tetrahedral
Answer (d) Tetrahedral
20. The correct order of lattice energies is
(a) NaCl > MgBr2 > CaO > Al2O3
(b) NaCl > CaO > MgBr2 > Al2O3
(c) Al2O3 > MgBr2 > CaO > NaCl
(d) Al2O3 > CaO > MgBr2 > NaCl
Answer (d) Al2O3 > CaO > MgBr2 > NaCl
21. The bond angle in water is
(a) 120°
(b) 107°
(c) 109.5°
(d) 104.5°
Answer (d) 104.5°
22. Which of the following molecule is planar?
(a) CH4
(b) C2H4
(c) NH3
(d) SiC14
Answer (b) C2H4
23. Sulphuric acid provides a simple example of
(a) Coordinate bonds
(b) Non-covalent compound
(c) Covalent ion.
(d) None of these.
Answer (d) None of these.
24. The interionic attraction depends on interaction of
(a) Solute solute
(b) The charges
(c) Solvent solvent
(d) Molecular properties
Answer (b) The charges
25. The pair of having similar geometry is
(a) BF,3,NH3
(b) H2O, C2H2
(c) CO2, SO2
(d) NH3 and PH3
Answer (d) NH3 and PH3
26. The strongest hydrogen bonding in its liquid phase is observed in
(a) H-F
(b) HI
(c) CH4
(d) PH3
Answer (a) H-F
27. Maximum polarity found in
(a) C-O
(b) C – Br
(c) C-S
(d) C-F
Answer (d) C-F
28. According to VSEPR theory, BeF, molecule is
(a) Planar
(b) Linear
(c) Tetrahedral
(d) Pyramidal.
Answer (b) Linear
29. The stability of an ionic compound ie because of
(a) Lattice energy
(b) Electron affinity
(c) Ionization energy
(d) Electronegativity.
Answer (a) Lattice energy
30. The small hybridised carbon atom bond is
(a) Propane
(b) Propene
(c) Butane
(d) Propyne
Answer (d) Propyne
31. The hybridisation of carbon in CO2 is
(a) sp
(b) Sp²
(c) Sp³
(d) None
Answer (a) sp
32. Which of the following has sp²-hybridisation?
(a) C2H6
(b) C2H4
(c) BeCl2
(d) C2H2
Answer (b) C₂H4
Also You Can Read Inorganic Chemistry MCQ
