Table of Contents
Inorganic Chemistry MCQs

Inorganic Chemistry MCQs with Answers PDF
Here is Best Inorganic Chemistry MCQs with Answers PDF for the 12th science class students and also B.sc chemistry students of all university. Here we are trying to provide you the important Chemistry MCQs which are Best for the NEET Exam, UGC NET and CSIR NET Exam etc.
Also Read Biology MCQ
MCQ on Atomic Structure PDF
Inorganic Chemistry MCQs with Answers PDF – 1
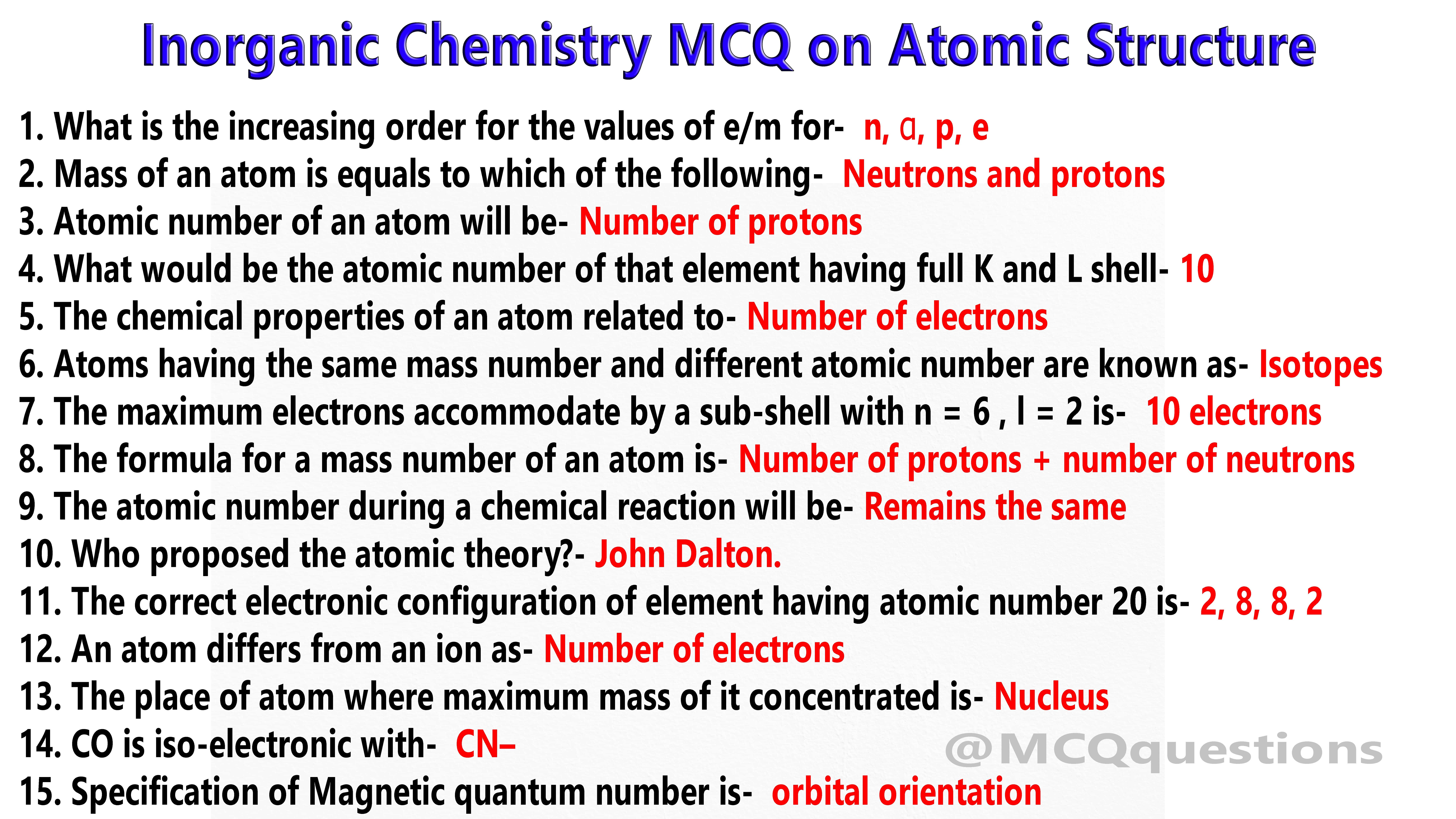
Inorganic Chemistry MCQ on Atomic Structure
1. What is the increasing order for the values of e/m for
(a) e, p, n, α
(b) n, p, e, α
(c) n, p, α, e
(d) n, α, p, e
Answer – (d) n, α, p, e
2. Mass of an atom is equals to which of the following
(a)Only protons.
(b)Only neutrons.
(c)Neutrons and protons.
(d)Protons and electrons.
Answer -(c)Neutrons and protons.
3. Atomic number of an atom will be
(a)Number of electrons.
(b)Number of protons.
(c)Number of electrons and protons.
(d)Number of protons and neutrons.
Answer -(b)Number of protons.
4. Nickel has atomic number 28. The correct electronic configuration is
(a)1s2 2s2 2p4 3s2 3p8 3d10
(b)1s2 2s2 2p6 3s2 3p6 3d8 4s2
(c)1s2 2s2 2p4 3s2 3p6 4s2
(d)1s2 2s2 3s2 3p8 3d10
Answer -(b)1s2 2s2 2p6 3s2 3p6 3d8 4s2
5. What would be the atomic number of that element having full K and L shell
(a)20
(b)14
(c)10
(d)16
Answer -(c)10
6. The chemical properties of an atom related to
(a)Number of protons.
(b)Number of electrons.
(c)Number of neutrons.
(d)None of these.
Answer -(b)Number of electrons.
7. Atoms having the same mass number and different atomic number are known as
(a)Isotopes.
(b)Isotones.
(c)Isobars.
(d)Isomers.
Answer -(a)Isotopes.
8. Which of the following quantum number helps to distinguish the electrons of the same orbitals ?
(a) Principal quantum number
(b) Azimuthal quantum number
(c) Magnetic quantum number
(d) Spin quantum number
Answer -(d) Spin quantum number
9. The maximum electrons accommodate by a sub-shell with n = 6 , l = 2 is
(a) 12 electrons
(b) 10 electrons
(c) 36 electrons
(d) 72 electrons
Answer -(b) 10 electrons
10. Given that the ionization enthalpy of hydrogen atom is 1.312 × 106 J mol-1. Then the required amount of energy for the excitation of the electron in the atom from n = 1 to n = 2 is
(a) 8.51 × 105 J mol-1
(b) 6.56 × 105 J mol-1
(c) 7.56 × 105 J mol-1
(d) 9.84 × 105 J mol-1 Inorganic Chemistry MCQs with Answers PDF
Answer-(d) 9.84 × 105 J mol-1
11. The formula for a mass number of an atom is
(a)Number of protons + number of electrons.
(b)Number of neutrons + number of electrons.
(c)Number of protons + number of neutrons.
(d) Non of these
Answer -(c)Number of protons + number of neutrons.
12. The atomic number during a chemical reaction will be
(a)Increases.
(b)Changes.
(c)Remains the same.
(d)Changes alternatively.
Answer -(c)Remains the same.
13. Who proposed the atomic theory?
(a)John Dalton.
(b)Robert Millikan.
(c)J. J. Thomson.
(d)Neils Bohr.
Answer -(a)John Dalton.
14. The correct electronic configuration of element having atomic number 20 is
(a)2, 6, 6, 2
(b)2, 8, 8, 2
(c)2, 4, 6, 2
(d)2, 4, 6, 2
Answer -(b)2, 8, 8, 2
15. An atom differs from an ion as
(a)Number of protons.
(b)Nuclear charge.
(c)Number of electrons.
(d)Mass number.
Answer -(c)Number of electrons.
16. The place of atom where maximum mass of it concentrated is
(a)Nucleus.
(b)Neutrons.
(C)Protons.
(d)Electrons.
Answer -(a)Nucleus.
17. CO is iso-electronic with
(a) O–2
(b) N+2
(c) CN–
(d) O+2
Answer -(c) CN–
18. Which of the following corresponds to the line spectrum of hydrogen obtained in the visible region of light?
(a) Lyman series
(b) Balmer series
(c) Paschen series
(d) Brackett series
Answer -(b) Balmer series
19. Which of following will be isolates, If the nitrogen atom had electronic configuration 1s², it would have energy lower than that of the normal ground state configuration 1s² 2s² 2p³, as the distance between electrons and the nucleus is too less. Yet, 1s² is not observed.
(a) Heisenberg’s Uncertainty Principle
(b) Hund’s rule
(c) Pauli Exclusion Principle
(d) Bohr postulate of stationary orbits
Answer-(c) Pauli Exclusion Principle
20. if the quantum numbers + 1/2 and – 1/2 for the electron spin it resembles
(a) rotation is clockwise and anticlockwise direction respectively
(b) rotation is anticlockwise and clockwise direction respectively
(c) m Is up and down respectively
(d) 2 quantum mechanical spin states which have no classified analogue
Answer -(d) 2 quantum mechanical spin states which have no classified analogue
21. What will be the possible energy value of the excited state for electron in Bohr orbitals of hydrogen is ,
(Given energy of electron in the first Bohr orbit of H atom is 13.6 eV.)
(a) – 3.4 eV
(b) -4.2eV
(c) – 6.8 eV
(d) + 6.8 eV
Answer-(a) – 3.4 eV
22. What will be the atomic number of the element , if the last entering electron in an element has quantum number n = 3, l = 2, m = + 2 and s = + 1 /2.
(a) 13
(b) 21
(c) 29
(d) 39
Answer- (c) 29
23. Given energy = 2.91 × 10-19], h = 6.36 × 10-34 Js, c = 3.0 × 108 m/s ,Then what will be the wavelength of light?
(a) 6.56 nm
(b) 656 nm
(c) 0.656 nm
(d) 65.6 nm
Answer- (b) 656 nm
24. Which of the following statement is explained by Bohr’s atomic model?
(a) the spectrum of hydrogen atom only
(b) The spectrum of an atom/ion having only one electron.
(c) the spectrum of hydrogen molecule
(d) the solar spectrum
Answer- (b) The spectrum of an atom/ion having only one electron.
25. Specification of Magnetic quantum number is
(a) orbital size
(b) orbital shape
(c) orbital orientation
(d) nuclear stability
Answer- (c) orbital orientation
26. Accurate set of quantum numbers belong to highest energy is
(a) n = 4, l = 0, m = 0, s = + 12
(b) n = 3, l = 0, m = 0, s = + 12
(c) n = 2, l = 1, m = 1, s = + 12
(d) n = 3, l = 2, m = 1, s = + 12
Answer- (d) n = 3, l = 2, m = 1, s = + 12
27. Write The correct sequence to label the subshells in an atom is
(a)S, p, d, f, g
(b)S, p, p, f, d
(c)S, s, p, p, d, f, g
(d)S, p, g, d, f
Answer – (a)S, p, d, f, g
28. Which rule will this configuration be violating,if electronic configuration for oxygen is written as 1s2 2s2 2p4.
(a)Aufbau’s principle.
(b)Hund’s rule.
(c)Pauli’s exclusion principle.
(d)None of the above.
Answer (d)None of the above.
29. If an atom has four unpaired electrons. Then total spin of electron is
(a)1
(b)2.5
(c)2
(d)4
Answer (c)2
30. The charge and mass ratio of cathod rays resembles to
(a) a-particles
(b) anode rays
(c) B-rays Inorganic Chemistry MCQs with Answers PDF
(d) protons.
Answer (c) B-rays
31.The nucleus of an atom has
(a) protons and neutrons.
(b) protons and electrons
(c) neutrons and electrons.
(d) protons, neutrons and electrons.
Answer (a) protons and neutrons.
32. Generally Cathode rays are known as
(a) protons
(b) electrons
(c) neutrons
(d) a-particles
Answer (b) electrons
33. Proton may be defined as
(a) an ionised hydrogen molecule
(b) an alpha-ray particle
(c) a fundamental particle
(d) nucleus of heavy hydrogen
Answer (c) a fundamental particle
34. The order of size of nucleus ia
(a) 10-⁸cm
(b) 10-¹³cm
(c) 10-¹⁰cm
(d) 10-¹⁸ cm.
Answer (b) 10-¹³cm
35. The electron having a mass of
(a) 9.11 × 10-²⁷ g
(b) 9.11 × 10-²³ g
(c) 9.11 x 10-²⁸g
(d) 9.11 × 10-³⁸ g.
Answer (c) 9.11 x 10-²⁸g
36. The charge on proton is
(a) 1.602 10-19 C
(b) 1.502 10-19 C
(c) 1.602 10-20 C
(d) 1.502 10-21 C.
Answer (a) 1.602 10-19 C
37. The average distance of electron from the nucleus within an atom is equals to the order of
(a) I cm
(b) 10¹³cm
(c) 10-⁸cm
(d) 10-¹²cm.
Answer (c) 10-⁸cm
38. The founder of anode rays
(a) Crookes
(b) Goldstein
(c) Thomson
(d) Chadwick.
Answer (b) Goldstein
39.The Canal rays are known as
(a) electrons
(b) neutrons
(c) protons
(d) positively charged ions.
Answer (d) positively charged ions.
40. Except which of the following, neutrons are
present in all atoms?
(a) H
(b) Ar
(c) He
(d) Ne
Answer (a) H
41. Cl (34,17)and Cl (37,17)differ from each other in number of
(a) nucleon
(b) positrons
(c) protons
(d) electrons.
Answer (a) nucleon
42. Isotones are those which have same
(a) atomic number
(b) mass number
(c) number of neutrons
(d) number of electrons.
Answer (c) number of neutrons
43. An isotone of Ge(76,32) is
(a) Ge(77,32)
(b) Kr (81,36)
(c) Se(77,34)
(d) As(77,33)
Answer (d) As(77,33)
44. The non-integral atomic masses having by many elements is due to
(a) they have isobars
(b) their isotopes have non-integral masses
(c) their isotopes have different masses
(d) the constituents, neutrons, protons and electrons. combine to give fractional masses.
Answer (c) their isotopes have different masses
45. The number of electrons in an atom is same as its
(a) atomic weight
(b) atomic number
(c) equivalent weight
(d) electron affinity.
Answer (b) atomic number
46. Neon iso-electronic with
(a) O
(b) Mg²+
(c) N-
(d) F-²
Answer (b) Mg²+
47. What is the ratio of charge to mass that was determined by J.J Thomson (in coulombs per gram)
(a) -1.76 10⁸coulombs/ g
(b) 1.76 10-⁸ coulombs/g
(c) -1.76x 10¹⁰ coulombs/g
(d) -1.76×10-¹⁰ coulombs /g.
Answer (a) -1.76 10⁸coulombs/ g
48. Rutherford’s scattering experiment explains about
(a) the size nucleus
(b) size electron
(c) radius of atom
(d) radius neutron
Answer (a) the size nucleus
49. According to Rutherford’s theory, which of the following statement is not true?
(a) alpha particles going near the nucleus are slightly deflected
(b) some of alpha -particles pass through the nucleus
(c) Most of the bita-particles pass through without deflection
(d) A few alpha particles are deflected back
Answer (b) some of alpha -particles pass through the nucleus
50. The mass of a neutron is
(a) same as that of a proton
(b) slightly less than proton
(c) slightly more than a proton
(d) same as that of an electron.
Answer (c) slightly more than a proton
51. Rutherford’s experiment of a particles showed for the first time that atom has
(a) nucleus
(b) electrons
(c) protons
(d) neutrons
Answer (a) nucleus
52. Which established the nuclear model of the atom used a beam of(basis of Rutherford’s theory)
(a) bita-particles, which impinged on a metal foil .
(b) Bita-rays, which impinged on a metal foil and ejected electron
(c) helium atoms, which impinged on a metal foil andst scallered
(d) helium nuclei, which impinged on a metal fail and got scallerod
Answer (d) helium nuclei, which impinged on a metal fail and got scallerod
MCQ on Quantum Numbers
1. For l = 1, the shape of the orbital is
(a) Unsymmetrical
(b) Spherically symmetrical
(c) Dumb-bell
(d) Complicated
Answer. (c) Dumb-bell
2. The principal quantum number represents
(a) Shape of an orbital
(b) Distance of electron from nucleus
(c) Number of electrons in an orbit
(d) Number of orbitals in an orbit
Answer. (b) Distance of electron from nucleus
3. How many electrons can be fit into the orbitals that comprise the 3rd energy level ?
(a) 2
(b) 8
(c) 18
(d) 32
Answer. (c) 18
4. If value of l = 0, the shape of the orbital is
(a) Rectangular
(b) Spherical
(c) Dumbbell
(d) Unsymmetrical
Answer. (b) Spherical
5. For n = 3, the number of possible orbitals are
(a) 1
(b) 3
(c) 4
(d) 9
Answer. (d) 9
6.When 3d orbital is complete, the new electron will enter the
(a) 4p-orbital
(b) 4f-orbital
(c) 4s-orbital
(d) 4d-orbital
Answer. (a) 4p-orbital
7. Total number of radial nodes of 3s and 2p orbitals are respectively.
(a) 2, 0
(b) 0, 2
(c) 1, 2
(d) 2, 1
Answer. (a) 2, 0
8. The mvr of an electron depends on
(a) Principal quantum number
(b) Azimuthal quantum number
(c) Magnetic quantum number
(d) All of these
Answer. (b) Azimuthal quantum number
9. The maximum energy is present in any electron at which area
(a) Nucleus
(b) Ground state
(c) First excited state
(d) Infinite distance from the nucleus
Answer. (d) Infinite distance from the nucleus
10.The number of orbitals for n = 4 will be
(a) 4
(b) 8
(c) 12
(d) 16
Answer. (d) 16
11.The two electrons in K sub-shell will differ in
(a) n
(b) l
(c) m
(d) s
Answer. (d) s
12.The element having atomic number 29 possess how many unpaired electrons in
d-orbitals
(a) 10
(b) 1
(c) 0
(d) 5
Answer. (c) 0
13. The similarities found between 2p and 3p orbital that is
(a) Shape
(b) Size
(c) Energy
(d) Value of n
Answer. (a) Shape
14. The azimuthal quantum number is related to
(a) Size
(b) Shape
(c) Orientation
(d) Spin
Answer. (b) Shape
15.Value of l for last electron of Na atom is
(a) 1
(b) 2
(c) 3
(d) 0
Answer. (d) 0
16. In d orbitals the maximum number of unpaired electron can be present is
(a) 1
(b) 3
(c) 5
(d) 7
Answer. (c) 5
17. The number of unpaired electrons in an O2 molecule is
(a) 0
(b) 1
(c) 2
(d) 3
Answer. (c) 2
18.The number of unpaired electron I the element for Z=29
(a) 1
(b) 3
(c) 4
(d) 2
Answer. (a) 1
19. Maximum how many quantum numbers may same by two electrons
(a) One
(b) Two
(c) Three
(d) Four
Answer. (c) Three
20. How many orbitals can have the following quantum numbers, n = 3, l = 1, ml = 0?
(a) 4
(b) 2
(c) 1
(d) 3
Answer: (c) 1
21. For the set of quantum numbers, n = 3, l = 1, m = -1, the maximum electrons will be
(a) 2
(b) 6
(c) 10
(d) 4
Answer: (a) 2
22. The maximum number of electrons that can fit in an orbital with n = 3 and l = 1?
(a) 14
(b) 6
(c) 10
(d) 2
Answer: (d) 2
23. Which of the following helps to determine the maximum number of electrons present in a subshell
(a) 2l + 1
(b) 2n2
(c) 4l + 2
(d) 4l – 2
Answer: (c) 4l + 2
24. Which of the following quantum numbers governs the spatial orientation of an atomic orbital?
(a) Magnetic quantum number
(b) Spin quantum number
(c) Azimuthal quantum number
(d) Principal quantum number
Answer: (a) Magnetic quantum number
25. Which one represents the three-dimensional shape of an atomic orbital
(a) Azimuthal quantum number
(b) Principal quantum number
(c) Spin quantum number
(d) Magnetic quantum number
Answer: (a) Azimuthal quantum number
26. The maximum number of orbitals present in a subshell can be represented by
(a) 2l + 1
(b) 2n2
(c) 4l + 2
(d) 4l – 2
Answer: (a) 2l + 1
27. For the orbitals with n = 2 and l = 1, the number of electrons that fit in it is
(a) 8
(b) 2
(c) 6
(d) 4
Answer: (c) 6
28. For l=3 the number of electrons will be
(a) 14
(b) 2
(c) 10
(d) 6
Answer: (a) 14
MCQ on Periodicity of Elements PDF
Inorganic Chemistry MCQs with Answers PDF – 2
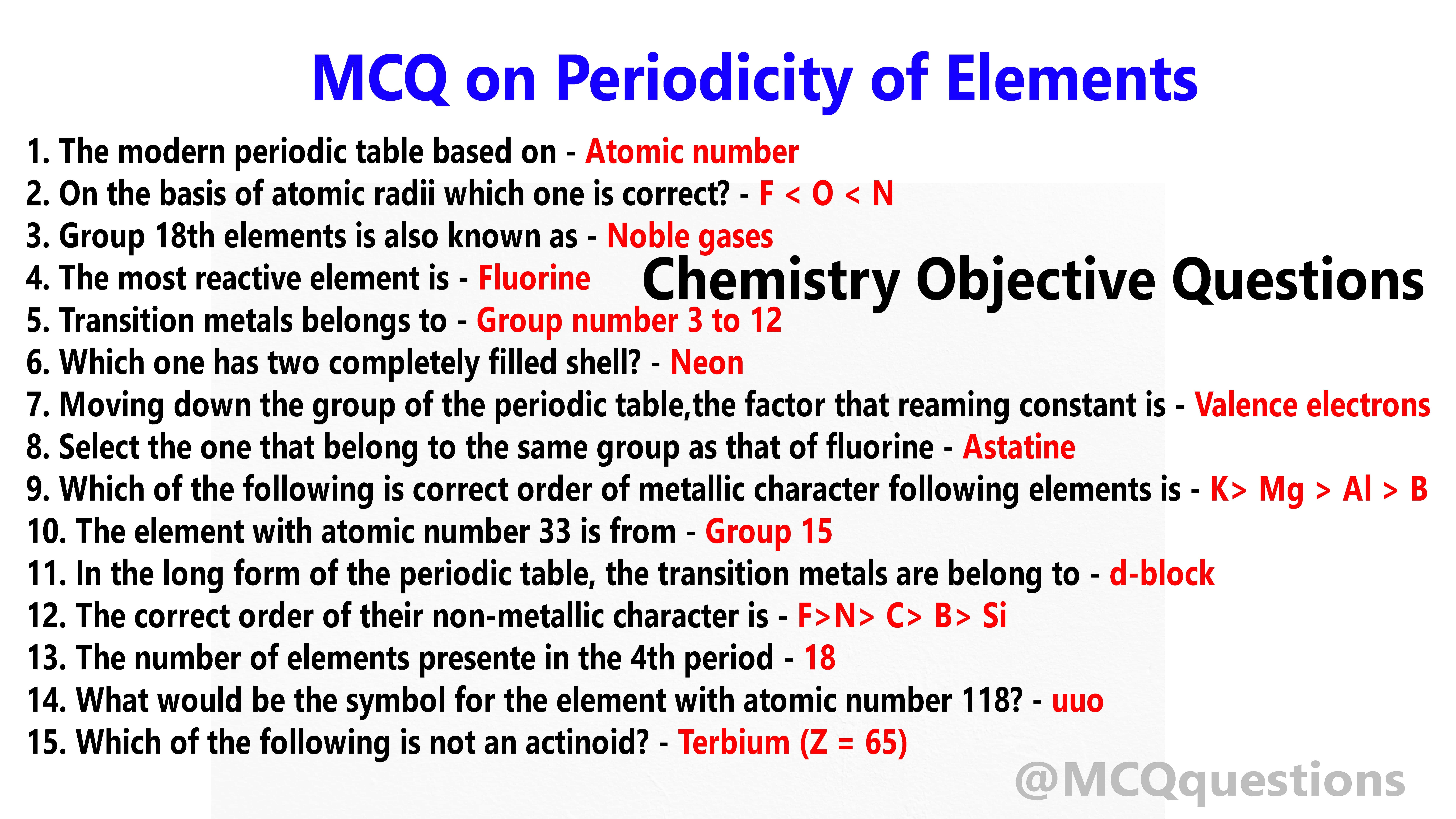
Inorganic Chemistry MCQ on Periodicity of Elements
1. The modern periodic table based on
(a) Atomic mass
(b) Atomic number
(c) Number of nucleons
(d) All of these
Answer b) Atomic number
2. The changes in electro positivity of elements on moving from left to right in a periodic table is
(a) Increase
(b) Decreases
(c) First increases than decreases
(d) First decreases than increases
Answer (b) Decreases
3. What is the position of the element in moder periodic table having electronic configuration 2, 8, 4. ?
(a) 4th group
(b) 2nd group
(c) 14th group
(d) 18th group
Answer (c) 14th group
4. On the basis of atomic radii which one is correct?
(a) O < F < N
(b) N < F < O
(c) O < N < F
(d) F < O < N
Answer (d) F < O < N
5. Group 18th elements is also known as
(a) Noble gases
(b) Alkali metals
(c) Alkali earth metals
(d) Halogens
Answer (a) Noble gases
6. The most reactive element is
(a) Oxygen
(b) Sodium
(c) Fluorine
(d) Magnesium
Answer (c) Fluorine
7. Transition metals belongs to
(a) Group number 1 to 2
(b) Group number 13 to 18
(c) Group number 3 to 12
(d) Group number 1 to 8
Answer (c) Group number 3 to 12
8. Which one has two completely filled shell?
(a) Helium
(b) Neon
(c) Calcium
(d) Boron
Answer (b) Neon
9. Moving down the group of the periodic table,the factor that reaming constant is
(a) Atomic radius
(b) Metallic character
(c) Number of shells in the atom
(d) Valence electrons
Answer (d) Valence electrons
10. Which of the following is not characteristic of d-block elements?
(a) They form coloured complexes
(b) They are generally paramagnetic
(c) Their ionization energies are very high
(d) They show variable oxidation states Inorganic Chemistry MCQs with Answers PDF
Answer (c) Their ionization energies are very high
11. Choose the wrong one.
(a) The properties of elements are the periodicity of their atomic number.
(b) metallic elements are more in number than non-metallic elements
(c) The first IE of elements along a period do not vary .
(d) For transition elements, the last electron enters into (n-3)d-subshell.
Answer (d) For transition elements, the last electron enters into (n-3)d-subshell
12. In the sixth period of the periodic table, we have
(a) Two s-block and six p-block elements
(b) Fourteen f-block elements
(c) ten d-block elements
(d) All the above.
Answer (d) All the above.
13. In the sixth period of the periodic table, 14 elements are placed in the third group. They are known as
(a) Alkali metals
(b) Rare earths
(c) Rare gases
(d) Alkaline carth metals
Answer (b) Rare earths
14. Select the one that belong to the same group as that of fluorine
(a) Astatine
(b) Cesium
(c) Radium
(d) Rubidium.
Answer (a) Astatine
15. Which of the following is correct order of metallic character following elements is
(a) B > Al> Mg > K
(b) Al> Mg > B> K
(c) Mg > Al> K > B
(d) K> Mg > Al > B.
Answer (d) K> Mg > Al > B.
16. Tik the incorrect statement in relation to ionization enthalpy?
(a) lonization enthalpy increases for each successive electron.
(b) The greatest IP found in core noble gas configuration.
(c) End of outer electron is marked by a big jump in ionization enthalpy.
(d) Removal of electron from lower n value is easier than from higher n value orbital.
Answer (d) Removal of electron from lower n value is easier than from higher n value orbital
17. The element with atomic number 33 is from
(a) Group 13
(b) Group 15
(c) Group 14
(d) Group 16.
Answer (b) Group 15
18. In the long form of the periodic table, the transition metals are belong to
(a) s-block
(b) p-block
(c) d-block
(d) f-block.
Answer (c) d-block
19. The correct order of their non-metallic character is
(a) B> C> Si>N> F
(b) Si > C> B>N> F
(c) F>N> C> B> Si
(d) F>N> C> Si > B
Answer (c) F>N> C> B> Si
20. Given energy of ground state electron of hydrogen -2.18 10-18 J. Calculate the ionization energy of atomic hydrogen in terms of J mol.
(a) 2.313 x 10⁶ J/mol
(b) 1.313 x 10⁶ J/mol
(c) 2.313 x 10-⁶ J/mol
(d) 4.313 x 10-⁶J/mol.
Answer (b) 1.313 x 10⁶ J/mol
21. Which of the following statements is incorrect?
(a) periodic table contains 7 period and 18 groups.
(b) The d-block has 8 columns.
(c) Each block contains a number of columns equal to the number of electrons.
(d) The block indicates l value for the last subshell.
Answer (b) The d-block has 8 columns.
22. The number of elements presente in the 4th period
(a) 8
(b) 10
(c) 18
(d) 13.
Answer (c) 18
23. Which of the following statements are incorrect?
(a) Helium is a noble gas.
(b) Chlorine has less negative EA than fluorine.
(c) s and p elements are Representative elements.
(d) In any period, atomic radius of alkali metals is the highest.
Answer (b) Chlorine has less negative EA than fluorine.
24. What would be the symbol for the element with atomic number 118?
(a) uuq
(b) uup
(c) uus
(d) uuo.
Answer (d) uuo.
25. Considering the elements F. CI, O and N, the correct order of oxidizing property is
(a) F> CI> 0>N
(b) F>O>CI > N
(c) CI>F>O>N
(d) O> F>N> CL
Answer (b) F>O>CI > N
26. Which of the following is not an actinoid?
(a) Curium (7- 96)
(b) Uranium (Z-92)
(c) Californium (Z = 98)
(d) Terbium (Z = 65).
Answer (d) Terbium (Z = 65).
27. Select the incorrect statement.
(a) Elements of group 16 are known as chalcogens.
(b) Actinoid elements are radioactive.
(c) Electronic configuration na belongs to s-block elements.
(d) Zn show most of the properties of transition elements.
Answer (d) Zn show most of the properties of transition elements.
28. Correct increasing metallic character order is
(a) P< Si < Na< Be < Mg
(b) Be< Mg<P < Na < Si
(c) Si <Be> Mg < Na < P
(d) P<Si < Be Mg < Na.
Answer (d) P<Si < Be Mg < Na.
29. Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
(a) Valence principal quantum number (n)
(b) Nuclear charge (Z)
(c) Nuclear mass
(d) Number of core electrons.
Answer (c) Nuclear mass
MCQ on Chemical Bonding PDF
Inorganic Chemistry MCQs with Answers PDF – 3
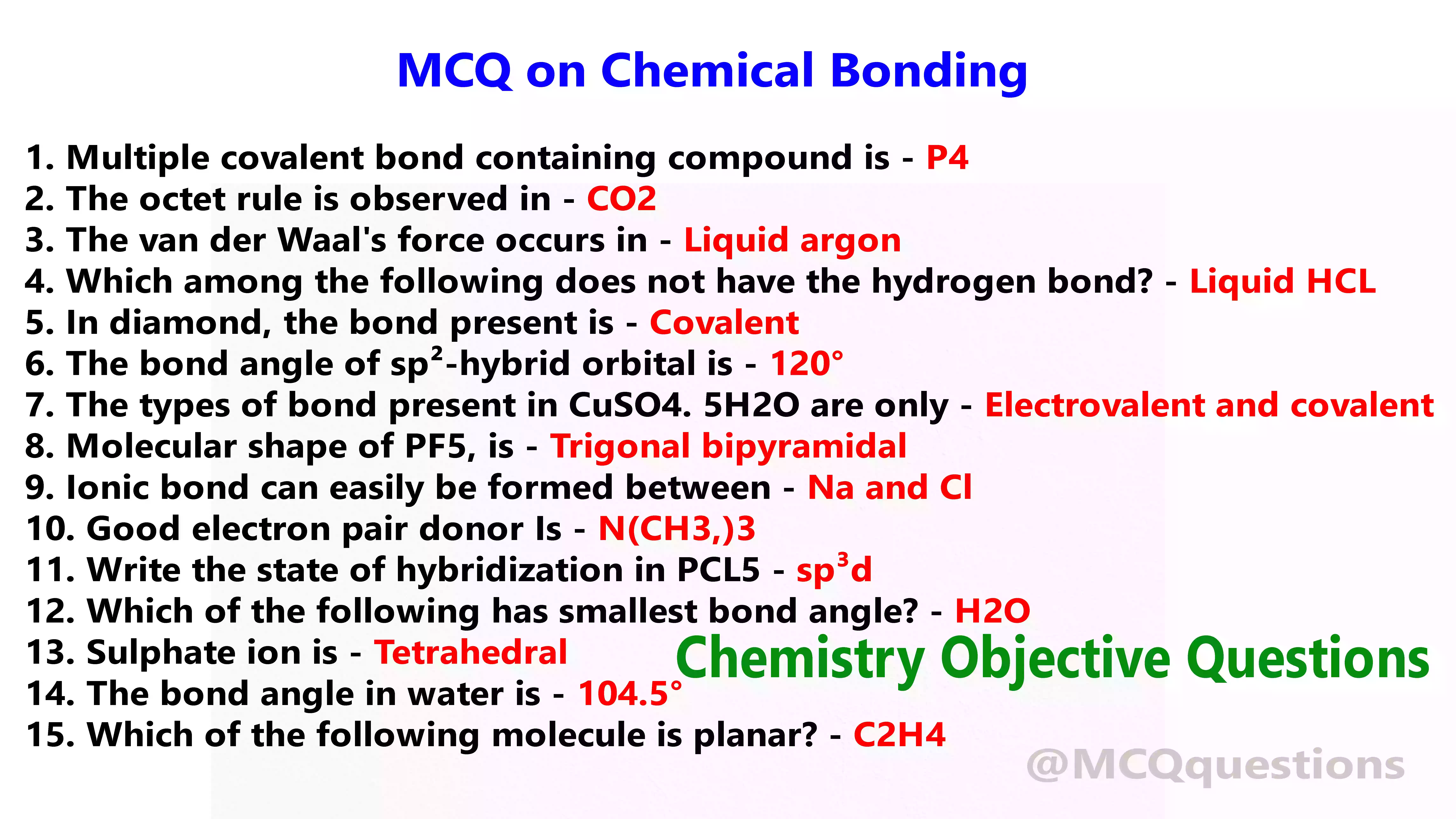
Inorganic Chemistry MCQ on Chemical Bonding
1. Multiple covalent bond containing compound is
(a) CH4
(b) S8
(c) P4
(d) H2
Answer (c) P4
2. The octet rule is observed in
(a) PCI5
(b) CO2
(c) BCI3
(d) SF6
Answer (b) CO2
3. The van der Waal’s force occurs in
(a) Liquid HF
(b) Liquid NH,
(c) Ice
(d) Liquid argon
Answer (d) Liquid argon
4. The compound containing coordinate bond is
(a) SO3
(b) H2SO4
(c) O3
(d) all of these
Answer (d) all of these
5. Which among the following does not have the hydrogen bond?
(a) Water
(b) Liquid NH
(c) Phenol
(d) Liquid HCL
Answer (d) Liquid HCL
6. Carbon tetrachloride has no dipole moment, because
(a) Similar sizes of carbon and chlorine atoms
(b) It is symmetrical tetrahedral structure
(c) It is planer structure
(d) None of these.
Answer (b) It is symmetrical tetrahedral structure
7. The tetrahedral structure is possessed by
(a) BF4-
(b) CH4
(c) NH4+
(d) all of these
Answer (d) all of these
8. In diamond, the bond present is
(a) Hydrogen
(b) Covalent
(c) Electrovalent
(d) Coordinate bond.
Answer (b) Covalent
9. The bond angle of sp²-hybrid orbital is
(a) 105°
(b) 120°
(c) 180°
(d) 109°28′
Answer (b) 120°
10. The types of bond present in CuSO4. 5H2O are only
(a) Electrovalent and covalent
(b) Electrovalent and coordinate covalent
(c) Electrovalent, covalent and coordinate-covalent
(d) Electrovalent, covalent and hydrogen.
Answer (a) Electrovalent and covalent
11. Molecular shape of PF5, is
(a) square planar
(b) Trigonal bipyramidal
(c) Tetrahedral three-dimensional
(d) Octahedral three-dimensional.
Answer (b) Trigonal bipyramidal
12. Ionic bond can easily be formed between
(a) H and O
(b) H and Cl
(c) H and N
(d) Na and Cl
Answer (d) Na and Cl
13. What happend in presence of more electronegative atom to the strength of ionic bond?
(a) Decreases
(b) Increases
(c) remains same
(d) Any one of these.
Answer (b) Increases
14. If we consider the ammonia molecule to be sp³ hybridised. the lone pair will occupy
(a) An s-orbital
(b) A p-orbital
(c) A sp²-hybridised orbital
(d) A sp³-hybridised orbital
Answer (d) A sp³-hybridised orbital
15. Strongest hydrogen bonds in vapour phase are found in
(a) HF—– HF
(b) HCI—–HCI
(c) HF—- HCI
(d) HF—-HI.
Answer (a) HF—– HF
16. Good electron pair donor
Is
(a) P(CH3)3
(b) PH3
(c) N(CH3,)3
(d) NH3
Answer (c) N(CH3,)3
17. Write the state of hybridization in PCL5
(a) dsp³
(b) sp³d
(c) dsp²
(d) sp³d²
Answer (b) sp³d
18. Which of the following has smallest bond angle?
(a) H2O
(b) NH3
(c) CH4
(d)CO2
Answer (a) H2O
19. Sulphate ion is
(a) square planar
(b) Pyramidal
(c) Rhombic
(d) Tetrahedral
Answer (d) Tetrahedral
20. The correct order of lattice energies is
(a) NaCl > MgBr2 > CaO > Al2O3
(b) NaCl > CaO > MgBr2 > Al2O3
(c) Al2O3 > MgBr2 > CaO > NaCl
(d) Al2O3 > CaO > MgBr2 > NaCl
Answer (d) Al2O3 > CaO > MgBr2 > NaCl
21. The bond angle in water is
(a) 120°
(b) 107°
(c) 109.5°
(d) 104.5°
Answer (d) 104.5°
22. Which of the following molecule is planar?
(a) CH4
(b) C2H4
(c) NH3
(d) SiC14
Answer (b) C2H4
23. Sulphuric acid provides a simple example of
(a) Coordinate bonds
(b) Non-covalent compound
(c) Covalent ion.
(d) None of these.
Answer (d) None of these.
24. The interionic attraction depends on interaction of
(a) Solute solute
(b) The charges
(c) Solvent solvent
(d) Molecular properties
Answer (b) The charges
25. The pair of having similar geometry is
(a) BF,3,NH3
(b) H2O, C2H2
(c) CO2, SO2
(d) NH3 and PH3
Answer (d) NH3 and PH3
26. The strongest hydrogen bonding in its liquid phase is observed in
(a) H-F
(b) HI
(c) CH4
(d) PH3
Answer (a) H-F
27. Maximum polarity found in
(a) C-O
(b) C – Br
(c) C-S
(d) C-F
Answer (d) C-F
28. According to VSEPR theory, BeF, molecule is
(a) Planar
(b) Linear
(c) Tetrahedral
(d) Pyramidal.
Answer (b) Linear
29. The stability of an ionic compound ie because of
(a) Lattice energy
(b) Electron affinity
(c) Ionization energy
(d) Electronegativity.
Answer (a) Lattice energy
30. The small hybridised carbon atom bond is
(a) Propane
(b) Propene
(c) Butane
(d) Propyne
Answer (d) Propyne
31. The hybridisation of carbon in CO2 is
(a) sp
(b) Sp²
(c) Sp³
(d) None
Answer (a) sp
32. Which of the following has sp²-hybridisation?
(a) C2H6
(b) C2H4
(c) BeCl2
(d) C2H2
Answer (b) C₂H4
